8A: What is Evidence Based Practice (EBP)?
This section covers:
What is ‘evidence’?
The term ‘evidence’ is used to describe results obtained from research studies or investigations to find answers to a clinical question. It includes findings from many different types of research investigations such as:
- randomised controlled trials
- case control studies
- time series and longitudinal studies
- descriptive or observational studies
- qualitative studies which may involve interviews or focus groups.
It also includes consensus statements which have been developed from panels of experts (e.g. European Wound Management Association Position Documents) for some situations where limited evidence is available.
The term ‘best evidence’ refers to an overall summary of combined results obtained after a rigorous evaluation of all research studies looking at treatments or care for a particular clinical problem of interest (e.g. looking for the best treatments for pressure injuries).
Evidence based practice
Evidence based practice has been described as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients” (Sackett et al. 1996).
The need for evidence to guide decisions about treatments or care is not new. Florence Nightingale used evidence from patient morbidity statistics to make decisions about the care provided to injured soldiers. Today many factors have pushed the need for evidence based practice to the fore, including rising costs of health care and the need to improve the quality of health care outcomes.
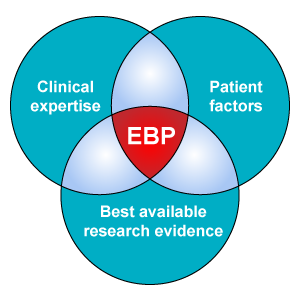
Evidence based practice does not simply mean applying the available research evidence. Rather, when making decisions on provision of care, evidence based practice involves consideration of:
- best available research evidence;
- clinical expertise and context; and
- client preferences.
This is represented in the following diagram:
 Top of page
Top of page
Evidence based guidelines
Evidence based guidelines or clinical practice guidelines are statements of recommended practice based on a systematic review of the best available evidence and expert opinion. A systematic review involves meticulously evaluating all research studies in a particular area and producing a summary of overall results. The need for systematic reviews and summaries is obvious when we consider:
- over 17,000 health science textbooks are published annually
- over 30,000 research journals are published annually
- more than 15 million papers are cited in MEDLINE (an international database of research journals).
If we just use one single study as evidence, then we may not get the whole picture. For example, single studies of fall prevention interventions have found they may increase patient falls, have no effect, or reduce falls by differing amounts - from 4% to 100%!
Research studies are evaluated according to:
- the strength of the evidence each study provides (i.e. the quality of a study and how well it reduced bias)
- the size of the effect obtained (i.e. how much difference did the treatment or intervention actually make? For example, was there a 20% difference in wound healing rates, or only 5%?)
- the relevance of any study results over different settings or contexts.
Guidelines are designed to translate the summarised research evidence into recommendations to guide clinical practice. They provide easily accessible information on treatments which have been shown to be effective and promote consistent treatment and continuity of care.
When referring to evidence based guidelines for direction, it is important to remember to:
- check the date the guidelines were published (i.e. guidelines should be updated every few years to accommodate updated research findings)
- consider the level of evidence supporting each recommendation
- realise that evidence based guidelines are only the first step in implementing evidence based practice (i.e. remember to consider the recommendations in combination with the available clinical expertise, the context of your particular situation and the client’s preferences for treatment).
Levels of evidence
Each recommendation provided in evidence based guidelines is accompanied by a classification (either A, B, C; or a Roman numeral system - I, II, III, IV, V, etc), which indicates the strength of evidence supporting that particular recommendation. An ‘A’ or ‘I’ is used for the highest or strongest level of evidence. There are many different classification systems for levels of evidence and each evidence based guideline will specify which system they are using and provide an explanation of the levels they use.
As an example, this project has used the Australian National Health and Medical Research Council levels of evidence as shown in the table below. These levels have been used for all the Guidelines Summaries listed on the Resources page.
| Level | Explanations |
| Level I | Evidence from a systematic review or meta-analysis of at least two level II studies. |
| Level II | Evidence from a well designed randomised controlled trial (for interventions), or a prospective cohort study (for prognostic studies). |
| Level III | Evidence from non-randomised studies with some control or comparison group (pseudo-randomised controlled trial; non-randomised experimental trial, cohort study, case-control study, time series studies with a control group; historical control study, retrospective cohort study). |
| Level IV | Evidence from studies with no control or comparison group. |
| An additional rating of Expert Opinion (EO) has been added, for guideline recommendations which are consensus statements provided by a National or International Panel of experts in the area. | |
For further information about levels of evidence, refer to: A guide to the development, evaluation and implementation of clinical practice guidelines (NHMRC).
Top of page


